Acella Pharmaceuticals, the maker of NP Thyroid, is facing a class action lawsuit over claims that the medication was defective, leading to inconsistent potency and adverse health effects. This lawsuit follows an NP Thyroid recall and raises concerns about the company’s quality control. For patients who relied on this thyroid medication, the lawsuit offers an opportunity to seek compensation for the harm caused.
At Sparrow, we specialize in guiding individuals through complex legal actions, including pharmaceutical disputes. Whether it’s issues similar to the NP Thyroid lawsuit, or cases involving product contamination recall, our expertise ensures that you are informed, prepared, and supported throughout the process so that you can focus on getting the justice and compensation you deserve.
Drawing from our decades of experience in class action settlements, we’ve developed a clear and comprehensive guide to help you navigate the NP Thyroid lawsuit. You’ll learn how to determine your eligibility, submit your claim, and understand the legal process.
Let’s get started!
Summary of the NP Thyroid Lawsuit
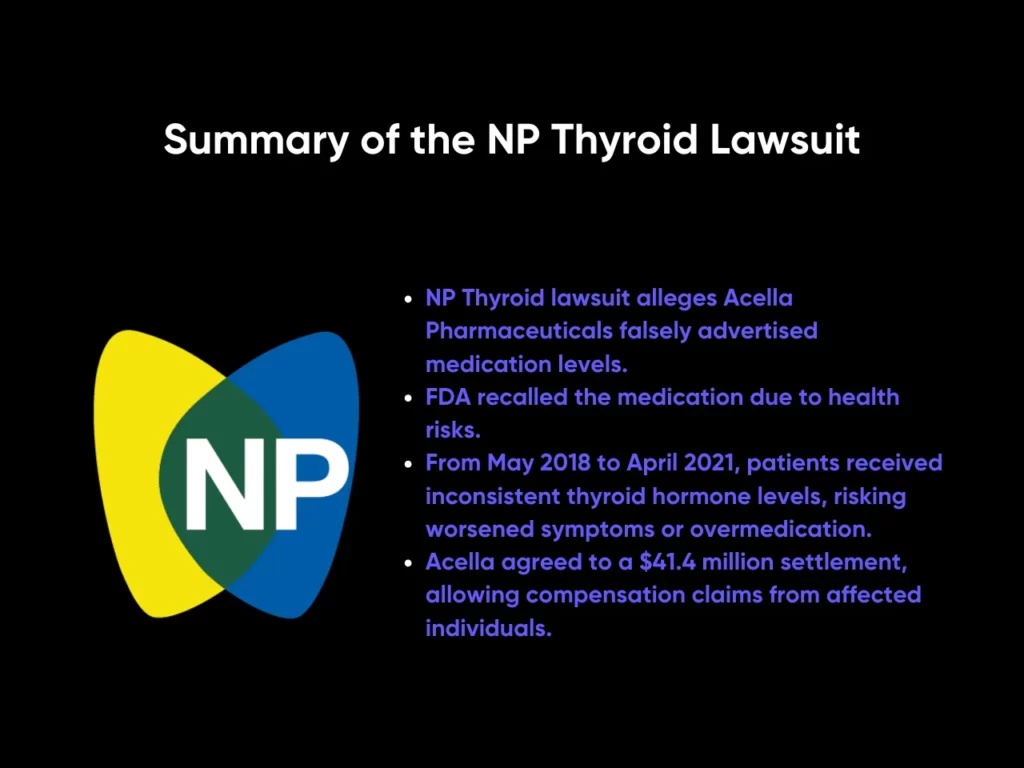
The NP Thyroid lawsuit alleges that Acella Pharmaceuticals, the manufacturer of NP Thyroid, falsely advertised their medication as containing specific and consistent levels of active ingredients necessary to treat hypothyroidism. The Food and Drug Administration (FDA) announced a recall of the thyroid medications because they pose health dangers to consumers.
Between May 2018 and April 2021, patients were dispensed medication that had either too much or too little of the active thyroid hormone. This inconsistency in dosage could result in dangerous health outcomes, including worsened hypothyroid symptoms or even overmedication.
Acella has not admitted fault but has agreed to a $41.4 million settlement to resolve the class action. The settlement allows individuals who were prescribed NP Thyroid tablets during the specified timeframe to file claims for compensation, regardless of whether the batch they received was part of an official voluntary recall.
NP Thyroid Company Background
Acella Pharmaceuticals is a pharmaceutical company known for producing NP Thyroid, a natural desiccated thyroid (NDT) prescription medications commonly prescribed for treating hypothyroidism.
Hypothyroidism (underactive thyroid) is a condition in which the thyroid gland does not produce enough thyroid hormone, resulting in symptoms like fatigue, weight gain, depression, and difficulty concentrating. Many thyroid patients opt for NP Thyroid over synthetic alternatives like levothyroxine, believing that the natural product offers a more balanced hormone profile.
There is reasonable risk of serious injury when pregnant women and their soon-to-be newborn infants are exposed to hypothyroidism. This may include early miscarriage, fetal hyperthyroidism, and impairments to fetal neural and skeletal development.
Meanwhile, elderly patients are susceptible to toxic cardiac manifestations of hyperthyroidism and a variety of cardiac diseases such as slow heart rate, cardiac arrhythmia, and cardiac pain. Other symptoms of hypothyroidism include adverse reactions such as hair loss, dry skin, unexplained weight gain, puffy face, and swelling of the thyroid gland.
Legal Grounds for the Lawsuit
Acella has been under scrutiny in recent years for failing to ensure the quality and consistency of NP Thyroid. The company issued three thyroid medication recalls in 2020 and 2021 after the FDA found significant quality control issues. These recalled lots, combined with the class action lawsuit, have raised serious concerns about Acella’s manufacturing practices and its commitment to patient safety. To date, Acella has received at least 43 reports of serious adverse events that could possibly be related to the recalls.
Allegations
The NP Thyroid lawsuit is based on several key legal claims, primarily focused on Acella’s failure to ensure the safety and effectiveness of its medication. These allegations include:

Negligence
Acella is accused of failing to maintain proper quality control standards during the manufacturing process. The company allegedly allowed defective batches of NP Thyroid to reach the market, with inconsistent levels of the active ingredient necessary for hypothyroid treatment. This negligence is supported by FDA inspections, which uncovered serious violations at Acella’s manufacturing facilities, including inadequate routine testing and quality assurance protocols.
False Advertising
Acella is also accused of falsely advertising NP Thyroid as containing specific and consistent amounts of active ingredients. Patients were misled into believing they were receiving reliable treatment, violating consumer protection laws designed to prevent companies from making false claims about product safety and efficacy.
Breach of Warranty
Acella allegedly breached both express and implied warranties by selling a product that did not meet the advertised standards of quality and safety. The company’s failure to deliver a medication that met basic safety requirements put patients’ health at risk, further violating consumer trust.
Patient Harm
Many patients experienced serious health issues that greatly reduced their quality of life as a result of receiving defective medication. There were also reports of worsened hypothyroidism symptoms or overmedication leading to anxiety and heart issues. These harmful side effects, the plaintiffs argue, could have been avoided had Acella adhered to proper manufacturing standards.
Delayed Recalls
Despite repeated warnings from the FDA, Acella allegedly delayed issuing recalls, allowing more patients to be exposed to defective batches. The lawsuit argues that Acella should have acted swiftly to remove the dangerous medication from circulation but instead failed to protect consumers in a timely manner.
Company’s Stance on the Case

While Acella Pharmaceuticals has agreed to the $41.4 million settlement, the company has not admitted to any wrongdoing. In public statements, Acella has maintained that the inconsistency in NP Thyroid was unintentional and that the company took steps to address the issue as soon as it became aware of the problem.
Acella’s defense has largely focused on the complexity of manufacturing natural desiccated thyroid products. The company argues that it followed industry standards and that any deviations in the hormone levels were minor and did not pose a significant risk to patients. However, the FDA’s findings and the recalls tell a different story and many patients who experienced negative health effects from the medication remain skeptical of Acella’s claims.
Settlement Details
Under the terms of the settlement, Acella Pharmaceuticals has agreed to set aside $41.4 million to compensate individuals who were prescribed NP Thyroid between May 12, 2018, and April 30, 2021. The settlement allows patients to receive compensation even if the medication they were prescribed was not part of the official recall.
- $10 compensation is available to individuals who file a claim without proof of purchase.
- Up to $50 can be claimed by individuals who provide documentation, such as pharmacy receipts, insurance statements, or other records that show they were prescribed NP Thyroid during the relevant period.
Timeline of Action
The NP Thyroid lawsuit progressed through a series of significant stages, beginning with initial recalls and culminating in a substantial settlement agreement. Below is a detailed breakdown of the major events that led to the resolution of the case:
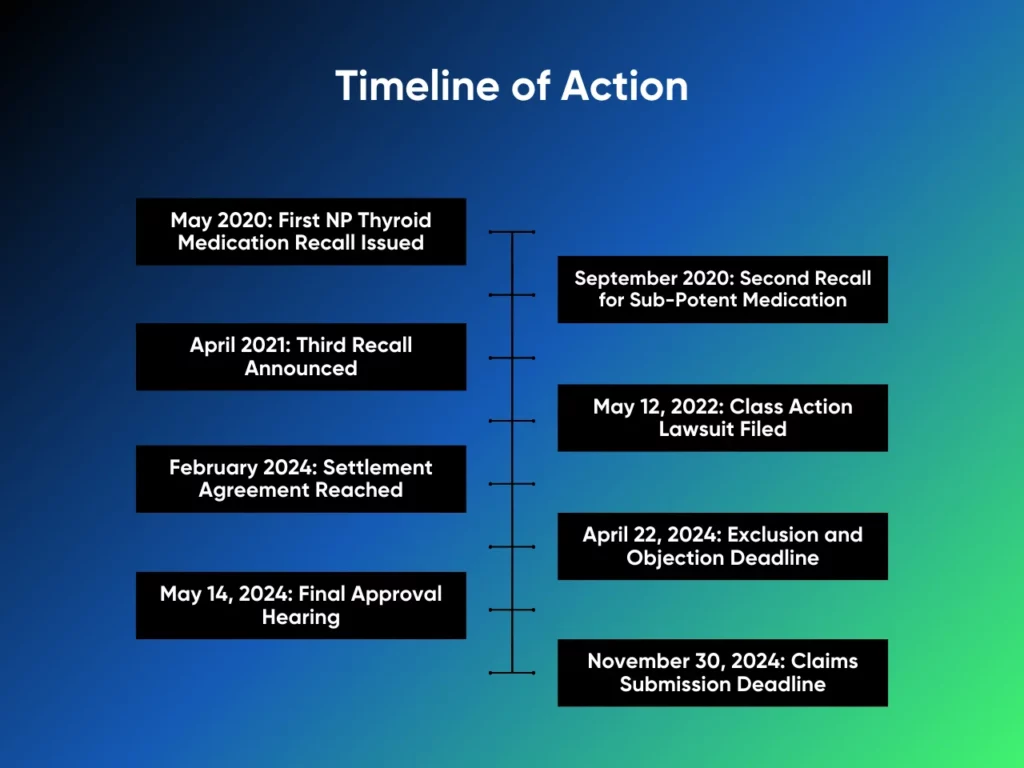
May 2020: First NP Thyroid Medication Recall Issued
Acella Pharmaceuticals initiated the first recall of NP Thyroid in May 2020. This recall was prompted by FDA investigations, which found that certain batches of the prescriptive medication contained super-potent doses of thyroid hormone, exceeding the safe and intended amounts. Patients taking these affected batches were at risk of serious health complications due to overmedication, prompting the recall of several lots.
September 2020: Second Recall for Sub-Potent Medication
In September 2020, Acella Pharmaceuticals issued a second recall, this time for sub-potent batches of NP Thyroid. The medication in these lots contained less than the required dose of the active ingredient, leading to potential undertreatment of patients. This recall raised additional concerns about the quality control measures in place at Acella’s manufacturing facilities.
April 2021: Third Recall Announced
A third recall followed in April 2021, covering additional lots of NP Thyroid that were found to be defective. This recall came after further FDA scrutiny and involved batches produced between March 2020 and March 2021. The repeated recalls led to widespread patient concerns and growing frustration among those who relied on NP Thyroid to manage their hypothyroidism symptoms.
May 12, 2022: Class Action Lawsuit Filed
The class action lawsuit, titled Faulkner, et al. v. Acella Pharmaceuticals, LLC, was officially filed on May 12, 2022, in the U.S. District Court for the Northern District of Georgia. The plaintiffs accused Acella of negligence, false advertising, and breach of warranty, citing the company’s failure to ensure the consistency and safety of NP Thyroid. The NP Thyroid lawsuit claimed that patients were dispensed a product that did not meet the advertised standards, resulting in economic loss and potential harm to their health.
February 2024: Settlement Agreement Reached
After months of negotiations and legal proceedings, Acella Pharmaceuticals agreed to a $41.4 million settlement in February 2024. While Acella did not admit to any wrongdoing, they agreed to a settlement to compensate patients affected by the inconsistencies in NP Thyroid. The agreement allowed individuals who had been prescribed NP Thyroid between May 12, 2018, and April 30, 2021, to file claims for compensation.
April 22, 2024: Exclusion and Objection Deadline
Patients wishing to opt out of the settlement or file objections to its terms must submit their requests by April 22, 2024. This is a critical date for anyone who does not wish to participate in the class action settlement and wants to preserve their right to pursue individual legal action against Acella.
May 14, 2024: Final Approval Hearing
A final approval hearing is scheduled for May 14, 2024. At this hearing, the court will decide whether the settlement terms are fair, reasonable, and adequate. If the judge grants final approval, the settlement will move forward, and eligible claimants will be able to receive their compensation.
November 30, 2024: Claims Submission Deadline
The final deadline to submit compensation claims is November 30, 2024. After this date, no further claims will be accepted, and eligible individuals who have not submitted a claim will forfeit their right to receive compensation.
Steps to Join the NP Thyroid Lawsuit
If you believe you’re eligible for compensation under the NP Thyroid lawsuit, following these steps will ensure you successfully file your claim and receive what you’re entitled to:
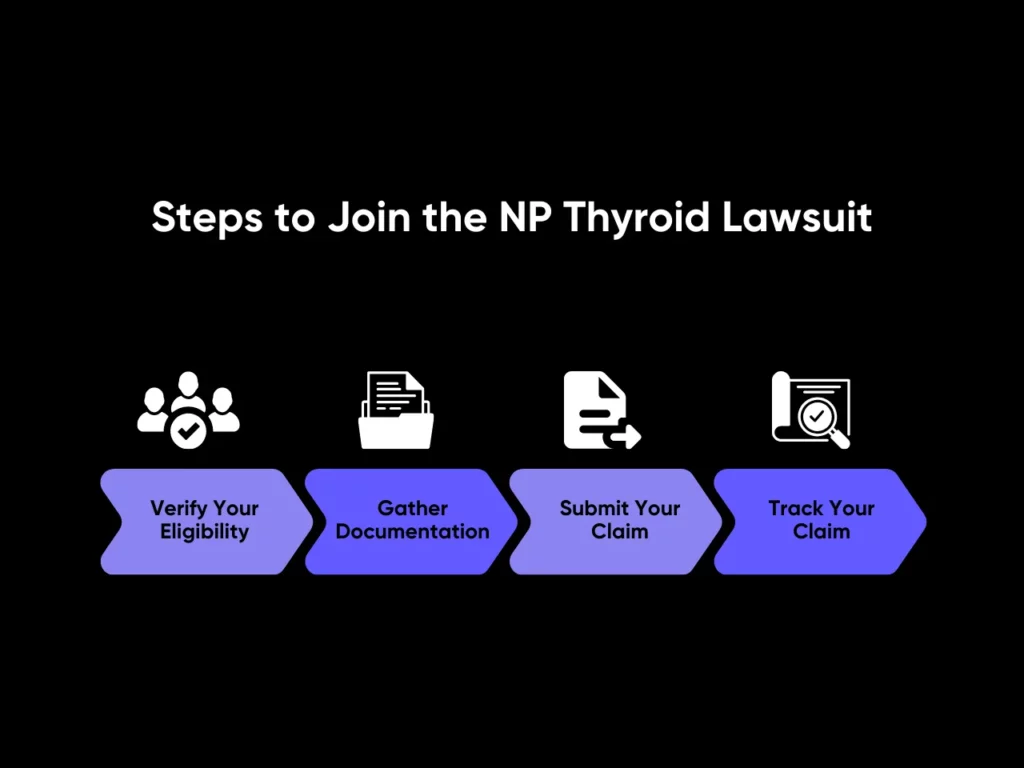
Verify Your Eligibility
Start by confirming that you were prescribed NP Thyroid between May 12, 2018, and April 30, 2021. You qualify to file a claim whether or not your medication was part of the official recall. If you’re unsure, check your pharmacy records or contact your healthcare provider for verification.
Gather Documentation
Although it’s not mandatory to submit documentation, having proof of your NP Thyroid prescription—such as pharmacy receipts, insurance statements, or medical records—can increase your compensation from $10 to $50. Even if you don’t have these documents, you can still file a claim and receive the $10 minimum compensation.
Submit Your Claim
Visit NPTSettlement.com to complete and submit your claim form online or download a form to mail it in. Ensure all information is accurate and that you attach any necessary documentation. The claim submission deadline is November 30, 2024.
Track Your Claim
Once your claim is submitted, stay informed on its progress. You can monitor your claim status via the settlement website or by contacting the AF9 Claims Administrator. This ensures you stay updated on any developments in the settlement process.
Key Takeaway
Wondering if you qualify for the NP Thyroid lawsuit? As more patients come forward with claims, this settlement offers a unique opportunity for those affected to seek compensation. Whether you experienced overmedication or worsening symptoms, it’s time to take action and explore your legal options.
Want to stay on top of the NP Thyroid lawsuit and other class actions? Sparrow is here to help you navigate the complexities of pharmaceutical claims and settlements. Whether you’re affected by defective medications like the Armour Thyroid recall or simply want to track the latest legal developments, we provide the guidance you need. Stay informed by following Sparrow’s blog for updates on ongoing cases and new settlement opportunities.

